The Space Surrounding The Nucleus Of An Atom Contains
Good luck to you.
The space surrounding the nucleus of an atom contains. What are found in the space surrounding the nucleus of an atom. In fact for all practical purposes the mass of the atom is the sum of the masses of the protons and neutrons. So your answer is b electrons.
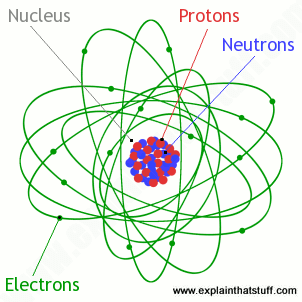
An electron is an elementary particle that is a fundamental constituent of matter having a negative charge and existing independently or as the component outside the nucleus of an atom. Each proton carries a positive charge and like charges repel each other. Not only is the nucleus very small but it also contains most of the mass of the atom.
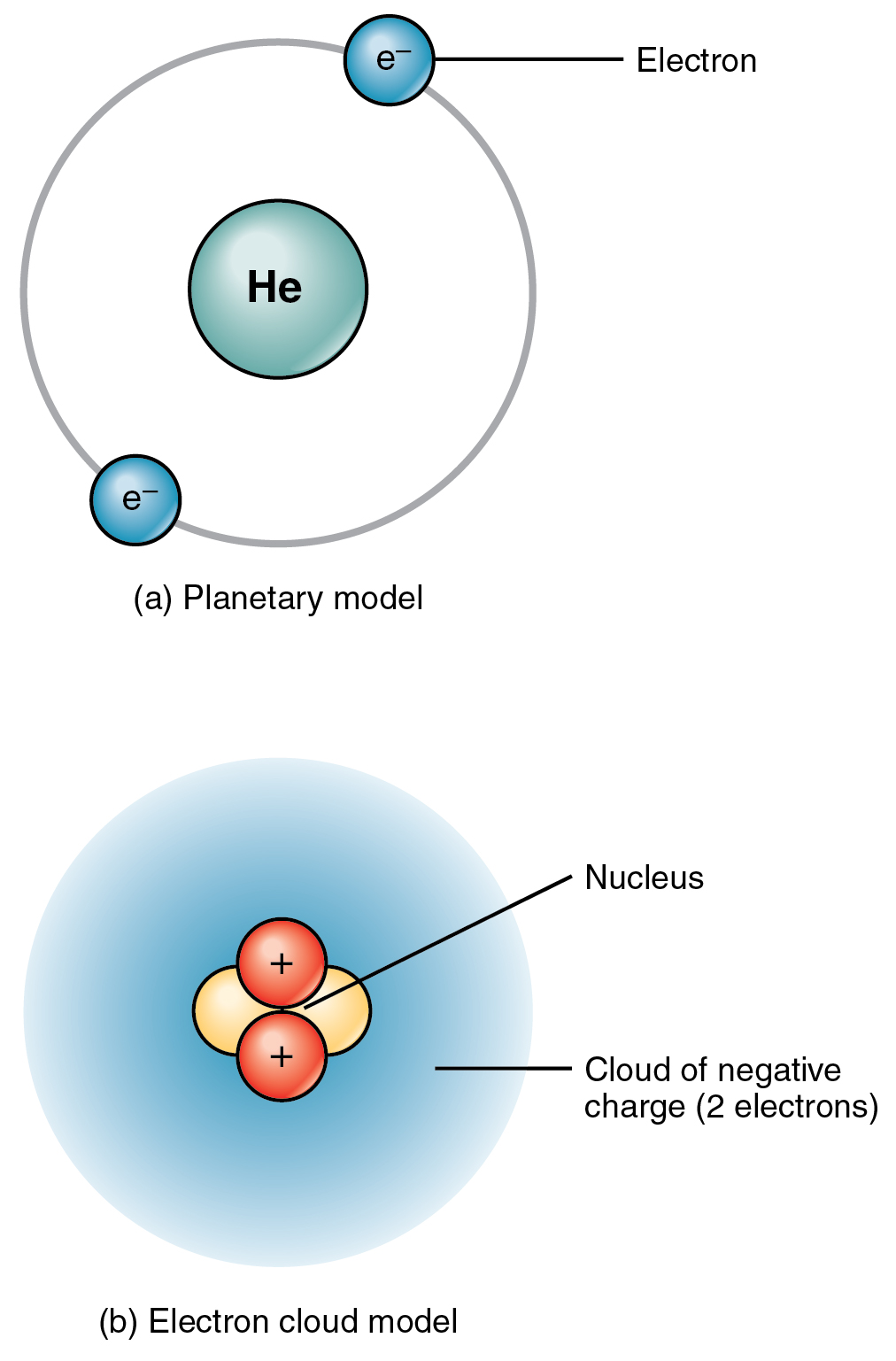
In the electron clouds surrounding the nucleus are electrons. The nucleus is very very small and very very dense when compared to the rest of the atom. What are found in the space surrounding the nucleus of an atom.
An atom has two subatomic particles which are neutrons and protons in the nucleus or center and electrons surrounding the nucleus in orbitals orbit like regions of space outside the nucleus. The nucleus contains neutrons and protons. The diameter of the atom is about 100 000 times bigger than the diameter of the nucleus.
Therefore most of the mass of an atom is contained in its nucleus. The protons of an atom are all crammed together inside the nucleus. Electrons move in orbits around the nucleus.
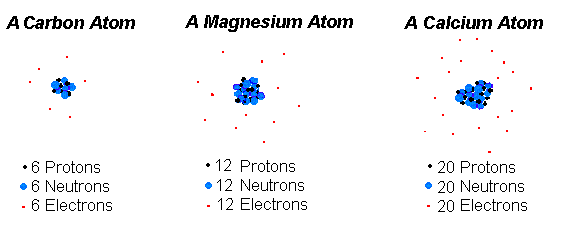
The protons and neutrons form a very small dense core known as the nucleus. Protons neutrons and electrons. Isotopes are tons of the same element with the same number of protons and a different number or.